Why Starting Material Quality Makes or Breaks Your Cell Therapy
Accelerating Your Path to Patients
In the race to bring transformative cell therapies to patients, there’s one critical element that often remains underestimated.
The quality and consistency of starting materials.
Reliable access to high-quality cellular starting material can become an unexpected bottleneck in development timelines and divert resources and focus away from a biotech’s key mission: developing groundbreaking therapies.
Additionally, cell therapies are moving up the treatment paradigm and patient populations are increasing, as such, the demand for efficient, standardized apheresis collections is increasing exponentially. This evolution presents both opportunity and challenge for developers navigating complex global regulatory landscapes.
Starting Material Quality is the Hidden Challenge in Cell Therapy Development
As we know, biological starting materials are inherently variable, which directly impacts:
-
Product consistency: when starting material varies, so does your final product
- Regulatory approval timelines: inconsistent inputs create data variability that regulators question
- Manufacturing efficiency: process optimizations based on variable inputs yield inconsistent results
- Patient outcomes: therapeutic potency depends on quality cellular foundations
But, cell therapy developers face a critical choice.
Either invest substantial resources in building, qualifying, and managing your own global apheresis networks, or partner with an established network built on standardized protocols and technologies.
There are some pitfalls to be aware of when it comes to a DIY approach. While blood banks and independent collection centers serve basic apheresis needs, they rarely address the full spectrum of challenges cell therapy developers face.
Most developers discover too late the complexities of navigating cross-border regulatory frameworks, establishing standardized protocols across multiple sites, and managing the intricate logistics of temperature-sensitive materials.
Europe's Largest Apheresis Network: A Competitive Advantage
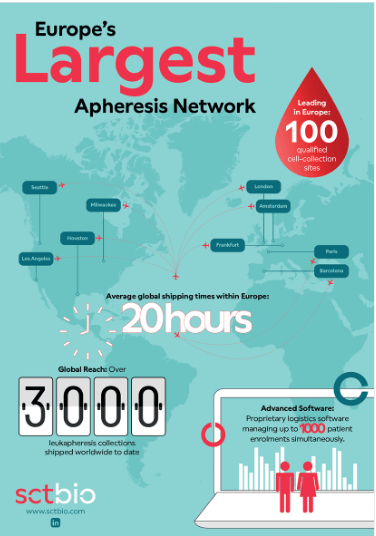
SCTbio maintains Europe's largest qualified apheresis network, comprising 100 qualified collection sites strategically positioned across key regions. This robust infrastructure represents more than just dots on a map—it embodies over 3,000 leukapheresis collections successfully shipped worldwide, with an average shipping time of just 20 hours.
Take a look at this infographic for more details.
But what truly differentiates SCTbio's network is the standardization at its core:
- Consistent collection protocols across all sites, minimizing variability
- Uniform equipment standards with flexibility for customer-specific requirements
- Streamlined regulatory compliance through centralized qualification, auditing and monitoring
- Training of apheresis site employees for specific collection process
- Proprietary logistics software managing up to 1,000 patient enrollments simultaneously
Ultimately, the value of a robust apheresis network isn't measured in site numbers or collection volumes, it's quantified in saved time, reduced complexity, and accelerated development timelines.
With cell therapies transforming treatment paradigms for serious diseases, every day saved in development translates to patients receiving life-changing treatments sooner. By reducing the lengthy timelines typically required to establish collection networks from scratch, SCTbio helps innovative therapies reach patients faster.
Discover how SCTbio's apheresis network can become your competitive advantage in cell therapy development and schedule a consultation with our apheresis specialists
This is the first installment in our thought leadership series on optimizing cell therapy starting materials. Look for upcoming articles on standardization approaches, auditing best practices, and enhancing the patient/donor experience.